ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed
outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Obesity has become one of the most pressing public health challenges in the United States and around the world. In the U.S., about 42% of adults are classified as obese, a figure that has nearly tripled over the past 50 years. Globally, over 650 million people are classified as obese, and this number continues to rise rapidly. Obesity increases the risk for many serious health conditions, including heart disease, type 2 diabetes, stroke, and certain cancers, making it a major contributor to premature death and disability worldwide.
Obesity is difficult to control due to several complex factors. It’s not simply a matter of overeating or lack of exercise; rather, it stems from a mix of genetics, hormonal imbalances, metabolism, and environmental factors, such as the easy availability of high-calorie foods and increasingly sedentary lifestyles. This complexity makes it challenging to address basic “eat less, move more” strategies. Additionally, many individuals struggle with yo-yo dieting, where initial weight loss is followed by weight regain once normal eating patterns resume. This cycle makes maintaining weight loss over the long term even more difficult. Furthermore, the medications currently available for weight loss often have limited effectiveness and can come with side effects, making them less appealing for long-term use. Most of these drugs only target specific aspects of obesity, like appetite suppression or metabolism, which doesn’t fully address the root causes of the issue.
Introduction
In recent years, GHK-Cu has garnered significant attention in the medical and scientific communities for its remarkable effects on wound healing, anti-aging, and anti-inflammatory properties. As research advances, GHK-Cu’s potential to combat inflammatory-based diseases has become particularly promising. Inflammation is a hallmark of many chronic diseases, including arthritis, COPD, and neurodegenerative disorders.
Understanding the molecular mechanisms of GHK-Cu and its therapeutic potential in targeting inflammatory pathways could be key in developing new treatments for these diseases. In this article, we will delve into the relationship between GHK-Cu and inflammatory-based diseases, exploring its mechanism of action, its role in reducing inflammation, and its future potential in applications.
One of the most intriguing aspects of Klotho is its association with aging. Klotho-deficient mice exhibit a spectrum of aging-related phenotypes, including vascular calcification, osteoporosis, cognitive decline, and reduced lifespan. Conversely, Klotho overexpression extends lifespan and mitigates age-related pathologies. The exact mechanisms by which Klotho influences aging are complex and multifactorial, involving oxidative stress, inflammation, and cellular senescence.
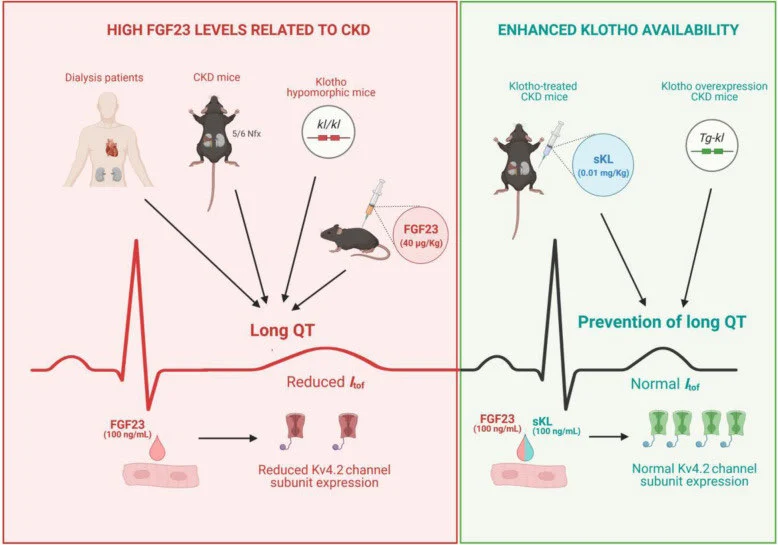
Klotho and Aging: The image illustrates the impact of FGF23 and klotho on heart health in CKD contexts. On the left, high FGF23 levels in dialysis patients and mice lead to Long QT Syndrome, marked by elongated QT intervals on ECGs, due to reduced Kv4.2 channel expression. On the right, increased klotho availability, either through treatment or genetic modification, prevents Long QT Syndrome, maintaining normal Kv4.2 channel expression and ECG patterns.
Klotho has been shown to possess antioxidant properties, reducing oxidative stress by increasing the expression of superoxide dismutase (SOD) and reducing reactive oxygen species (ROS) levels. Additionally, Klotho modulates inflammatory responses by inhibiting the nuclear factor-kappa B (NF-κB) signaling pathway, which is involved in the transcription of pro-inflammatory cytokines. These anti-oxidative and anti-inflammatory effects contribute to the protective role of Klotho against age-related diseases.
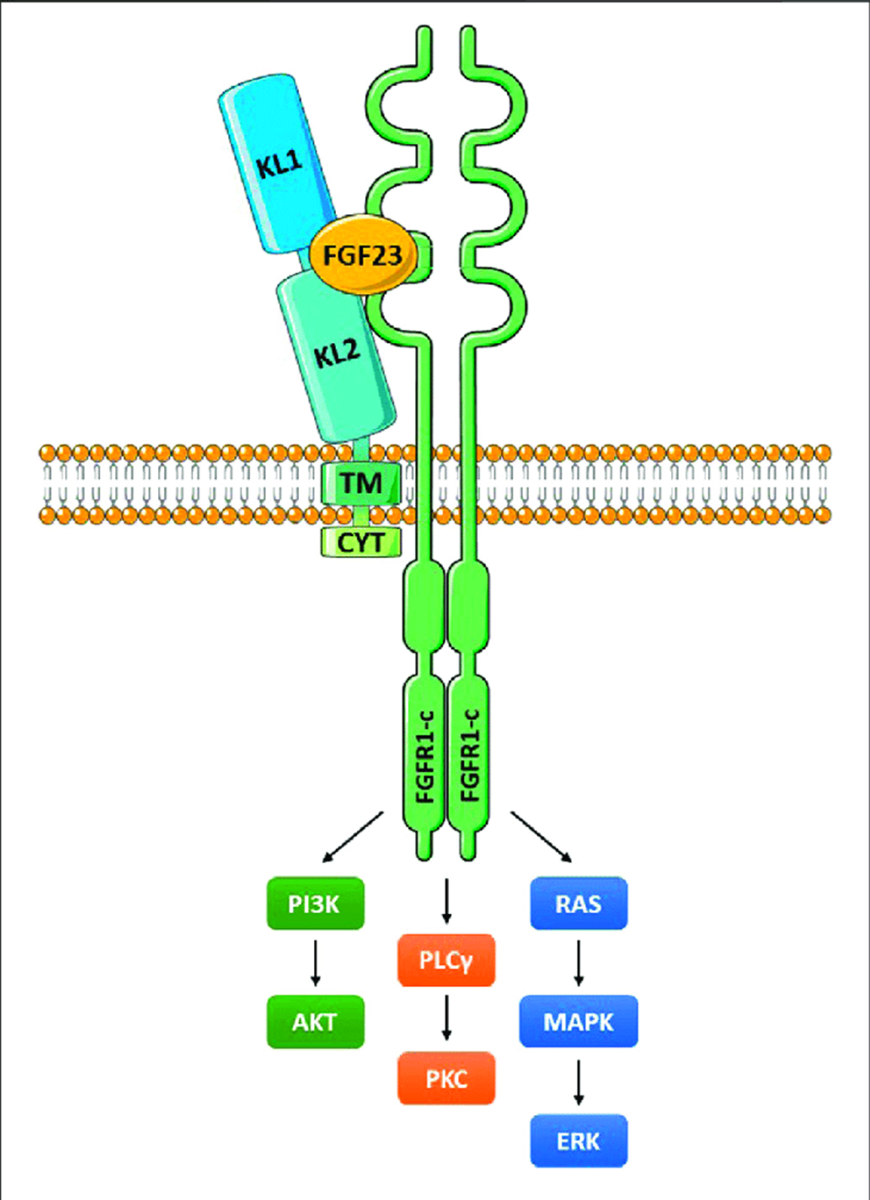
Cellular senescence, a state of irreversible cell cycle arrest, is a hallmark of aging. Klotho has been implicated in the regulation of cellular senescence through its effects on telomere maintenance and the insulin/IGF-1 signaling pathway. Klotho enhances telomerase activity, which is crucial for telomere elongation and genomic stability. Moreover, Klotho attenuates insulin/IGF-1 signaling, which has been associated with increased lifespan and reduced cellular senescence.
Klotho in Chronic Kidney Disease
Chronic kidney disease (CKD) is characterized by a progressive loss of renal function and is associated with reduced Klotho levels. The decline in Klotho expression in CKD contributes to disturbances in mineral metabolism, vascular calcification, and increased cardiovascular risk. Klotho deficiency in CKD exacerbates hyperphosphatemia and disrupts the delicate balance of the Klotho-FGF23 axis, leading to adverse outcomes.
Therapeutic Potential of Klotho
Given its diverse roles in aging, metabolism, and disease, Klotho has garnered attention as a potential therapeutic target. Strategies to enhance Klotho expression or mimic its activity are being explored for their therapeutic potential in age-related diseases, CKD, and other conditions.
PEPITEM (Peptide Inhibitor of Trans-Endothelial Migration) was first identified in 2015 by researchers at the University of Birmingham. New research published in Cell Reports Medicine demonstrates for the first time that PEPITEM could be utilized as a novel early clinical intervention to mitigate the effects of age-related musculoskeletal diseases. The data indicates that PEPITEM enhances bone mineralization, formation, and strength, effectively reversing bone loss in animal models.
This research was supported by significant grants from the Medical Research Council and the Lorna and Yuti Chernajovsky Biomedical Research Foundation, which invests in research for developing new targeted medicines to improve health outcomes. Additional funding was provided by the British Society for Research on Ageing and Versus Arthritis.
Bone undergoes constant formation, reformation, and remodeling throughout life, with up to 10% of human bone being replaced annually. This process involves a complex interplay between osteoblasts, which form bone, and osteoclasts, which break down bone. Disruptions to this finely tuned process are responsible for diseases such as osteoporosis and rheumatoid arthritis, characterized by excessive bone breakdown, or ankylosing spondylitis, marked by abnormal bone growth.
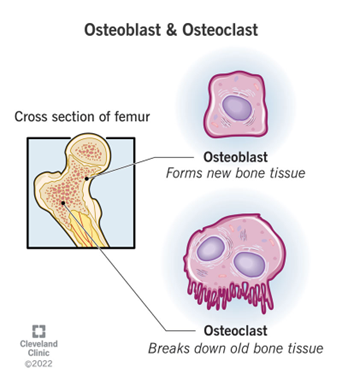
PEPITEM’s ability to enhance bone formation without adversely affecting osteoclast activity presents a promising therapeutic strategy. By promoting osteoblast activity and reducing osteoclast numbers via specific signaling pathways, PEPITEM has shown potential in increasing bone density and strength, suggesting its effectiveness as an early intervention for age-related bone diseases. This advancement could offer a new approach to maintaining bone health and preventing fractures, addressing a significant need in the treatment of osteoporosis and other musculoskeletal disorders.
Current osteoporosis therapies, such as bisphosphonates, primarily target osteoclasts to prevent further bone loss. While there are new anabolic agents available that promote new bone formation, their clinical use is limited. For example, teriparatide (parathyroid hormone, or PTH) is effective for only 24 months, and romosozumab (an anti-sclerostin antibody) has been linked to cardiovascular events. These limitations show the urgent need for developing new therapies to stimulate bone repair, especially in age-related musculoskeletal diseases like osteoporosis.
To address this need, a team of researchers led by Dr. Helen McGettrick and Dr. Amy Naylor, along with Dr. Jonathan Lewis and Ms. Kathryn Frost from the Institute of Inflammation and Ageing at the University of Birmingham, and Dr. James Edwards from the Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences at the University of Oxford, have been investigating the therapeutic potential of PEPITEM (Peptide Inhibitor of Trans-Endothelial Migration) in these conditions. Their research aims to explore whether PEPITEM can effectively stimulate bone repair and provide a new therapeutic avenue for treating osteoporosis and other age-related musculoskeletal disorders. By enhancing bone mineralization, formation, and strength, PEPITEM offers an exciting alternative that could overcome the limitations of existing therapies and significantly improve patient outcomes.
“To verify that VIP delays IVDD in vivo, we administrated exogenous VIP to mice with lumbar instability-induced lumbar IVDD via a sustained release pump (1004 W, RWD, Shenzhen, China) for continuous 4 weeks. The VIP-treated group’s Pfirrmann grade scores were significantly lower than those of the IVDD group according to MRI four weeks after surgery (Figure 7A,B). VIP could slow the progression of lumbar instability-induced IVDD in vivo. Consistently, histological analysis demonstrated a relatively vague frontier between NP tissue and AF tissue, as well as evidently reduced NP tissue and glycosaminoglycan in IVDD group. However, in VIP-treated group, there was still a little part of NP tissue within pathological IVD (Figure 7C–E). Likewise, IF staining unveiled that the expression of ACAN was partly reversed in VIP-treated group, accompanied by less expression of MMP3, compared with the IVDD group (Figure 7F,G). In addition, IHC staining in VIP-treated group, the expression of phosphorated FRS2 was also elevated (Figure 7C). Overall, these results indicated the therapeutical potential of VIP addition to ameliorate IVDD.”
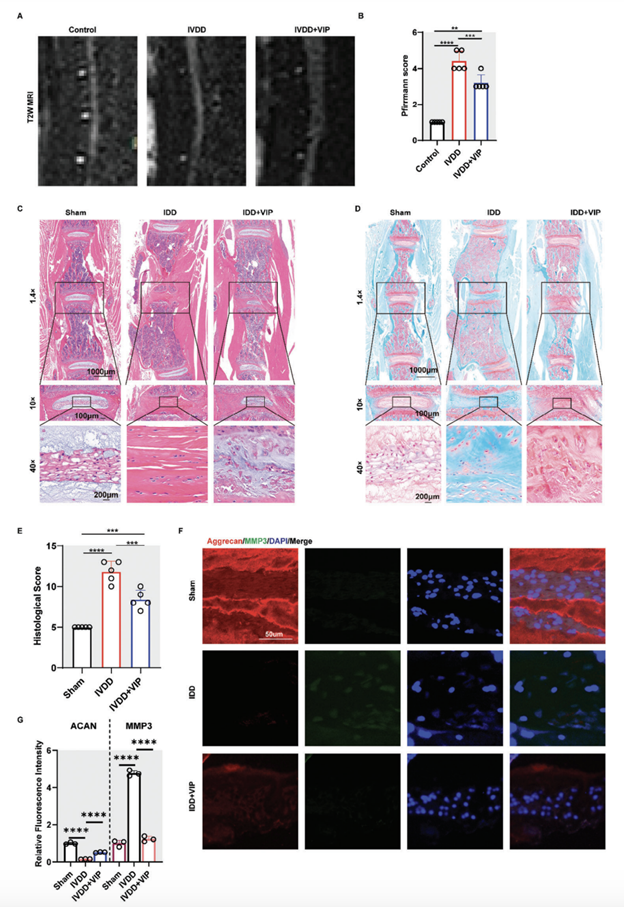
Figure 7. VIP alleviated lumbar instability-induced intervertebral disc degeneration by modulating FGFR2/FRS/AKT pathways in vivo A) Representative MRI images of mice lumbar spine in control, IVDD, and IVDD+VIP groups. B) Degenerated score of lumbar IVD in mice model based on T2-weighed MRI. C) Representative images of H&E staining for lumbar IVD in mice model from control, IVDD, and IVDD+VIP groups. D) Representative images of SOFG staining for lumbar IVD in mice model from control, IVDD, and IVDD+VIP groups. E) Histological score of lumbar IVD in mice model from control, IVDD, and IVDD+VIP groups. F,G) Representative images of IF staining and quantification results for aggrecan and MMP3 of lumbar IVD in mice model from control, IVDD, and IVDD+VIP groups. All data are shown as the mean ± SD. Statistical significance was determined by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns: not significant.
Receive all the latest information on events, sales, & offers.
T: 1-800-986-6401
Monday - Friday 9AM - 4PM PST
service@peptidesciencesmail.com
Mon - Fri / Except Holidays Orders placed and paid after 12 PM PST are shipped the following business day
P-Sciences
2831 St. Rose Pkwy Suite 362
Henderson, NV 89052
USA
© 2024
PeptideSciences.com. All Rights Reserved.
All products on this site are for Research, Development use only. Products are Not for Human consumption of any kind.
The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease.
Peptide Sciences is a chemical supplier. Peptide Sciences is not a compounding pharmacy or chemical compounding facility as defined under 503A of the Federal Food, Drug, and Cosmetic act. Peptide Sciences is not an outsourcing facility as defined under 503B of the Federal Food, Drug, and Cosmetic act.